1. Global Pharmaceutical Compliance Software Market Definition
The global Pharmaceutical Compliance Software market size is estimated to be $466.07 million in 2025 with a CAGR of 9.91% from 2025 to 2030.
Pharmaceutical Compliance Software is a specialized category of software solutions designed to help pharmaceutical companies navigate the complex landscape of regulatory requirements. These solutions play a crucial role in managing compliance with various regulatory bodies, such as the FDA and EMA, by streamlining processes related to product information management, electronic registration, and overall regulatory compliance. The software enables companies to maintain high standards of quality and safety while reducing the risk of non-compliance, which can lead to severe financial and legal consequences.
Global Pharmaceutical Compliance Software Market Value (M USD) in 2025
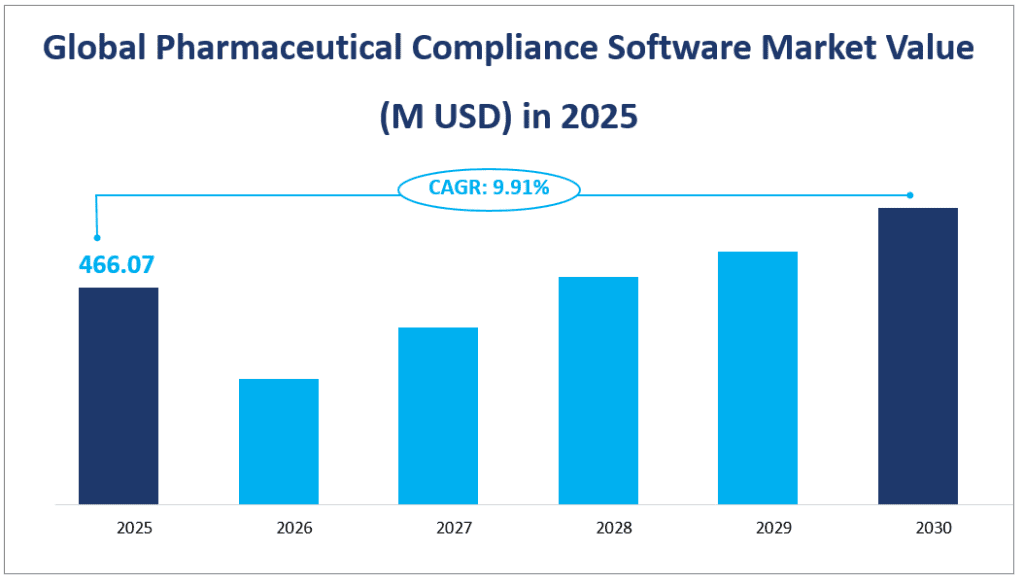
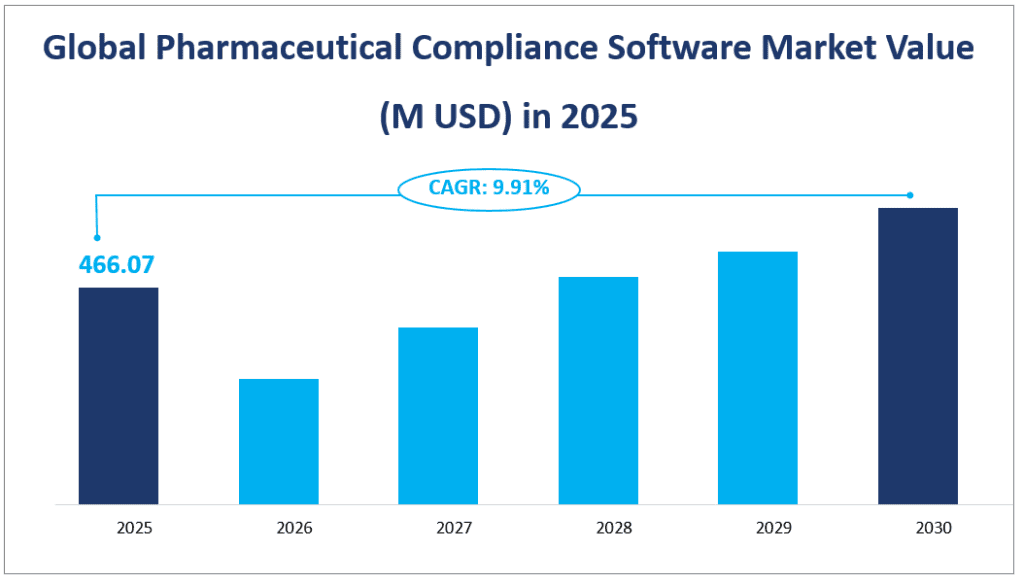
2. Driving Factors of the Pharmaceutical Compliance Software Market
Increasing Regulatory Complexity: The pharmaceutical industry is one of the most heavily regulated sectors, with stringent requirements for product safety, quality, and compliance. Regulatory bodies such as the FDA, EMA, and others continuously update and enforce new regulations. Pharmaceutical Compliance Software helps companies stay abreast of these changes, ensuring that they remain compliant and avoid costly penalties.
Technological Advancements: The integration of advanced technologies like artificial intelligence (AI), machine learning, and big data analytics has significantly enhanced the capabilities of compliance software. These technologies enable real-time monitoring, predictive analytics, and automated reporting, making compliance management more efficient and effective.
Globalization and Market Expansion: As pharmaceutical companies expand their operations globally, the need for robust compliance solutions becomes more critical. Compliance software helps companies manage regulatory requirements across multiple jurisdictions, ensuring consistency and compliance in their global operations.
Growing Demand for Transparency: Stakeholders, including consumers, healthcare providers, and regulatory bodies, increasingly demand transparency in pharmaceutical operations. Compliance software facilitates this by providing detailed records and reports, enhancing trust and credibility.
Cost and Efficiency Benefits: Compliance software reduces the administrative burden associated with manual compliance processes, leading to significant cost savings. It also improves operational efficiency by automating repetitive tasks and providing real-time insights, allowing companies to focus on core business activities.
3. Limiting Factors of the Pharmaceutical Compliance Software Market
High Initial Investment and Maintenance Costs: Implementing Pharmaceutical Compliance Software requires significant upfront investment in terms of purchasing, installation, and customization. Additionally, ongoing maintenance and updates can be costly, especially for small and medium-sized enterprises (SMEs).
Data Security and Privacy Concerns: The pharmaceutical industry deals with highly sensitive data, including intellectual property and patient information. Ensuring the security and privacy of this data is paramount. Cloud-based solutions, despite their benefits, raise concerns about data breaches and unauthorized access, which can deter some companies from adopting them.
Resistance to Change: Traditional manual processes are deeply ingrained in many pharmaceutical organizations. Transitioning to new software solutions requires significant changes in workflow and employee training, which can be met with resistance. This resistance can slow down the adoption of compliance software.
The complexity of Integration: Integrating compliance software with existing IT infrastructure can be challenging, especially for large organizations with multiple legacy systems. Compatibility issues and the need for extensive customization can prolong the implementation process and increase costs.
Regulatory Variations: Regulatory requirements vary significantly across different regions and countries. Developing and maintaining compliance software that meets these diverse requirements can be complex and resource-intensive, potentially limiting the market’s growth.
4. Pharmaceutical Compliance Software Market Segment
Product Types
Cloud-based solutions are rapidly gaining traction in the pharmaceutical industry due to their flexibility, scalability, and cost-effectiveness. By 2025, the market size for Cloud-Based Pharmaceutical Compliance Software is projected to reach $352.77 million. This segment is characterized by its ability to provide real-time access to data and compliance tools, allowing organizations to manage their regulatory requirements efficiently without the need for extensive hardware infrastructure.
On-premise solutions, while less flexible than their cloud counterparts, remain a crucial segment in the market. By 2025, the market size for On-Premise Pharmaceutical Compliance Software is expected to be $113.31 million. These solutions are deployed on the organization’s servers, providing a high level of control over data security and privacy. This is particularly important for pharmaceutical companies dealing with sensitive information such as intellectual property and patient data.
In terms of market share, Cloud-Based Pharmaceutical Compliance Software holds a dominant position, accounting for approximately 75.69% of the total market in 2025. This is significantly higher than the 24.31% market share held by On-Premise Solutions. The preference for cloud-based solutions is driven by their ability to provide real-time compliance management, scalability, and cost savings.
Applications in the Pharmaceutical Compliance Software Market
Product Information Management (PIM) is a critical application within the Pharmaceutical Compliance Software market. By 2025, the market size for PIM is expected to reach $329.49 million. PIM solutions help pharmaceutical companies manage their product lifecycle, from development to market launch, ensuring that all product-related information is accurate, up-to-date, and compliant with regulatory standards.
Pharmaceutical Electronic Registration is another important application within the market. By 2025, the market size for this application is projected to be $136.58 million. Electronic registration solutions facilitate the submission of regulatory documents and reports to authorities such as the FDA and EMA. These solutions ensure that all submissions are accurate, complete, and compliant with regulatory requirements, reducing the risk of delays and rejections.
In terms of market share, Product Information Management holds the largest portion, accounting for approximately 70.70% of the total market in 2025. This is significantly higher than the 29.30% market share held by Pharmaceutical Electronic Registration. The dominance of PIM is driven by its critical role in managing the product lifecycle and ensuring compliance across various stages of product development and distribution.
Market Size and Share by Segment
| Market Size (M USD)in 2025 | Market Share in 2025 | ||
| By Type | Cloud-Based Pharmaceutical Compliance Software | 352.77 | 75.69% |
| On-Premise Pharmaceutical Compliance Software | 113.31 | 24.31% | |
| By Application | Product Information Management | 329.49 | 70.70% |
| Pharmaceutical Electronic Registration | 136.58 | 29.30% |
5. Regional Pharmaceutical Compliance Software Market
North America remains the largest regional market for Pharmaceutical Compliance Software, with a projected market size of $193.73 million in 2025. This region’s dominance is attributed to its advanced healthcare infrastructure, stringent regulatory environment, and high adoption of digital technologies. Pharmaceutical companies in North America, particularly in the United States and Canada, are increasingly relying on compliance software to manage complex regulatory requirements and ensure data integrity. The region’s market share is expected to remain steady at around 41.57% of the global market in 2025.
Europe is the second-largest regional market, with an estimated market size of $123.29 million in 2025. The European market is characterized by strong regulatory frameworks enforced by the European Medicines Agency (EMA) and other national bodies. Compliance software adoption is driven by the need to manage regulatory submissions, quality control, and data management across diverse markets. Key countries contributing to the European market include Germany, France, the UK, Italy, and the Nordic countries. Europe’s market share is projected to be 26.45% in 2025, with a steady growth trajectory.
The Asia-Pacific region is the fastest-growing market for Pharmaceutical Compliance Software, with a projected market size of $121.56 million in 2025. This region’s growth is driven by rapid economic development, increasing pharmaceutical production, and the adoption of advanced technologies. Countries such as China, Japan, South Korea, and India are leading the charge, with significant investments in compliance infrastructure. The Asia-Pacific region is expected to account for 26.08% of the global market in 2025, reflecting its growing importance in the global pharmaceutical industry.
Latin America is a smaller but growing market, with an estimated market size of $16.64 million in 2025. The region’s growth is driven by increasing pharmaceutical production and the need for regulatory compliance in countries such as Mexico and Brazil. The Middle East & Africa region is projected to have a market size of $10.86 million in 2025. This region’s growth is driven by increasing healthcare investments and regulatory reforms in countries such as Turkey, Saudi Arabia, and the UAE. The region’s market share is expected to be 2.33% in 2025, with a focus on improving compliance standards and adopting advanced software solutions.
Global Pharmaceutical Compliance Software Market Share by Region in 2025
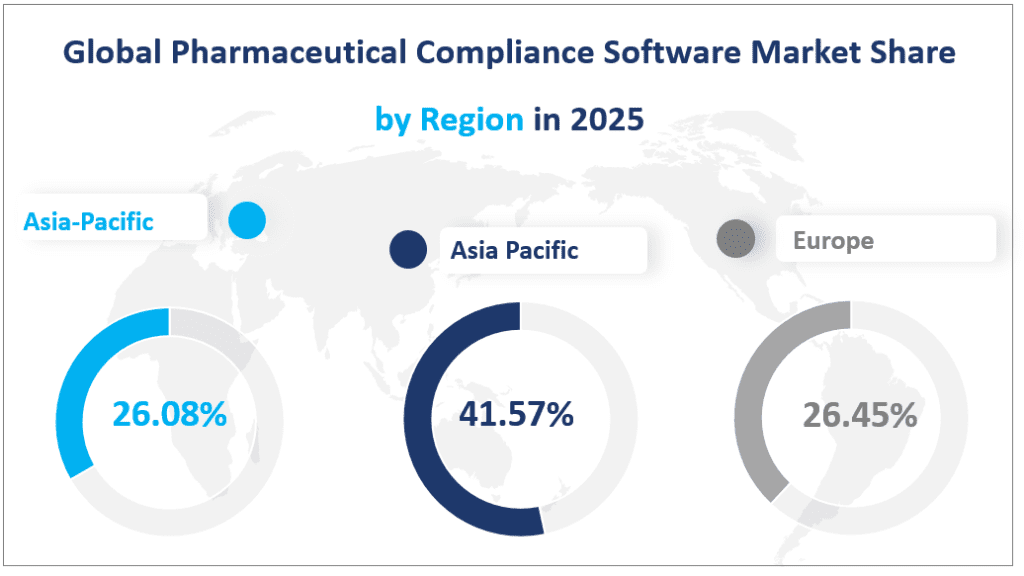
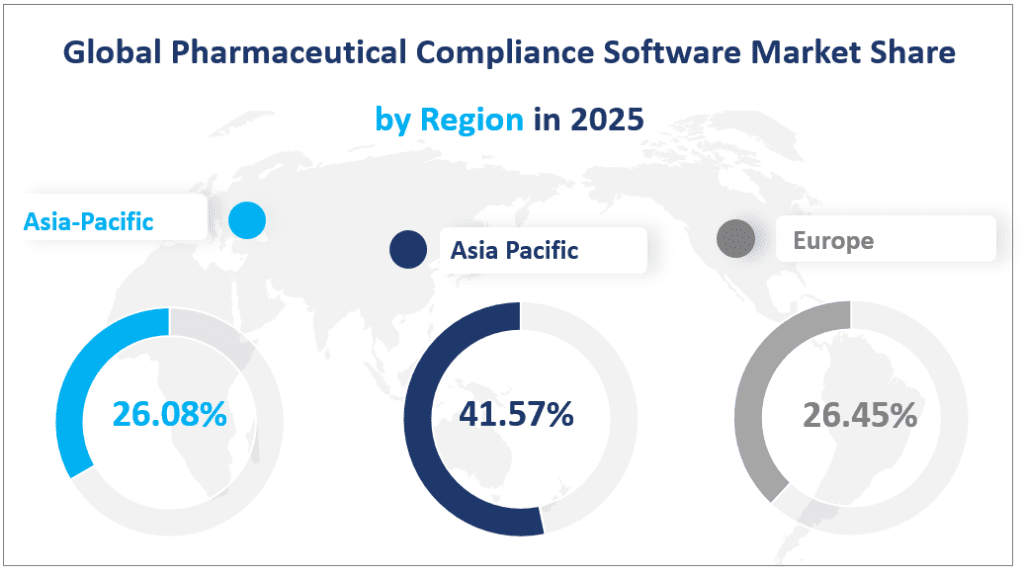
6. Top 3 Companies in the Pharmaceutical Compliance Software Market
Company Introduction and Business Overview:
Wolters Kluwer is a global leader in professional information, software solutions, and services for various sectors, including healthcare, compliance, and regulatory affairs. Founded in 1836 and headquartered in the Netherlands, Wolters Kluwer has a long history of providing reliable and comprehensive solutions to help organizations navigate complex regulatory landscapes.
Products Offered:
Wolters Kluwer’s flagship product in the compliance software space is the MediRegs Pharmaceutical Regulation Suite. This comprehensive tool provides real-time access to regulatory documents, compliance updates, and enforcement activities from government agencies such as the FDA. Key features include daily updates, personalized alerts, and a centralized repository of regulatory content, making it an essential tool for pharmaceutical companies.
In 2022, Wolters Kluwer’s revenue in the Pharmaceutical Compliance Software market was $31.27 million.
Company Introduction and Business Overview:
ACUTA, an IQVIA Company, is a leading provider of Regulatory Information Management (RIM) solutions and services for the life sciences industry. Founded in 2012 and headquartered in the United States, ACUTA offers integrated regulatory solutions, business process consulting, and technology services to help pharmaceutical companies manage their compliance needs effectively.
Products Offered:
ACUTA’s compliance software solutions are powered by the IQVIA CORE, an integrated platform that combines advanced analytics, global data, and deep domain expertise. The platform offers innovative pharmacovigilance solutions, enabling companies to achieve their safety and surveillance goals efficiently. Key features include real-time monitoring, risk management, and compliance analytics.
In 2022, ACUTA’s revenue in the Pharmaceutical Compliance Software market was $15.39 million.
Company Introduction and Business Overview:
Bwise, now part of SAI Global, is a leading provider of integrated governance, risk, and compliance (GRC) platforms. Founded in 1994 and headquartered in the Netherlands, Bwise offers comprehensive solutions to help organizations manage risk, create trust, and achieve business resilience through a unified approach to compliance.
Products Offered:
Bwise’s compliance software, now known as SAI360, is a highly configurable platform that helps organizations transform their compliance programs. Key features include unified risk management, automated regulatory content feeds, and centralized compliance repositories. The platform also offers robust tools for conducting assessments, remediating gaps, and maintaining audit readiness.
In 2022, Bwise’s revenue in the Pharmaceutical Compliance Software market was $15.63 million.
Major Players
| Company Name | Headquarters | Area Served |
| Wolters Kluwer | Netherlands | Worldwide |
| ACUTA | USA | Worldwide |
| Bwise | Netherlands | Worldwide |
| Qordata | USA | Worldwide |
| Lachman Consultant Services | USA | Mainly in North America and Europe |
| Ideagen | UK | Worldwide |
| MasterControl | USA | Mainly in North America, Europe, and Oceania |
| Sparta Systems | USA | Mainly in North America, Europe, and Oceania |
| Intagras | USA | Mainly in USA |
| LogicGate | USA | Mainly in North America, Europe |
1 Report Overview
1.1 Study Scope
1.2 Market Analysis by Type
1.2.1 Global Pharmaceutical Compliance Software Market Size Growth Rate by Type: 2017 VS 2021 VS 2027
1.2.2 Cloud-Based Pharmaceutical Compliance Software
1.2.3 On-Premise Pharmaceutical Compliance Software
1.3 Market by Application
1.3.1 Global Pharmaceutical Compliance Software Market Share by Application: 2017 VS 2021 VS 2027
1.3.2 Different Application of Pharmaceutical Compliance Software
1.4 Study Objectives
1.5 Years Considered
2 Global Growth Trends
2.1 Global Pharmaceutical Compliance Software Market Perspective (2017-2027)
2.2 Pharmaceutical Compliance Software Growth Trends by Region
2.2.1 Pharmaceutical Compliance Software Market Size by Regions: 2017 VS 2021 VS 2027
2.2.2 Pharmaceutical Compliance Software Historic Market Size by Regions (2017-2022)
2.2.3 Pharmaceutical Compliance Software Forecasted Market Size by Regions (2022-2027)
2.3 Pharmaceutical Compliance Software Industry Dynamic
2.3.1 Market Trends
2.3.2 Market Drivers
2.3.3 Market Challenges
2.3.4 Market Restraints
3 Competition Landscape by Key Players
3.1 Global Pharmaceutical Compliance Software Players by Revenue
3.1.1 Global Pharmaceutical Compliance Software Revenue by Players (2017-2022)
3.1.2 Global Pharmaceutical Compliance Software Revenue Market Share by Players (2017-2022)
3.2 Global Pharmaceutical Compliance Software Market Share by Company Type (Tier 1, Tier 2 and Tier 3)
3.3 Players Covered: Ranking by Pharmaceutical Compliance Software Revenue
3.4 Global Pharmaceutical Compliance Software Market Concentration Ratio
3.4.1 Global Pharmaceutical Compliance Software Market Concentration Ratio (CR5 and HHI)
3.5 Pharmaceutical Compliance Software Key Players Head office
3.6 Pharmaceutical Compliance Software Key Players Established Time
3.7 Pharmaceutical Compliance Software Key Players Area Served
3.8 Mergers & Acquisitions, Expansion Plans
4 Pharmaceutical Compliance Software Breakdown Data by Type
4.1 Global Pharmaceutical Compliance Software Historic Market Size by Type (2017-2022)
4.2 Global Pharmaceutical Compliance Software Forecasted Market Size by Type (2022-2027)
5 Pharmaceutical Compliance Software Breakdown Data by Application
5.1 Global Pharmaceutical Compliance Software Historic Market Size by Application (2017-2022)
5.2 Global Pharmaceutical Compliance Software Forecasted Market Size by Application (2022-2027)
6 North America
6.1 North America Pharmaceutical Compliance Software Market Size (2017-2027)
6.2 North America Pharmaceutical Compliance Software Market Size by Type
6.2.1 North America Pharmaceutical Compliance Software Market Size by Type (2017-2022)
6.2.2 North America Pharmaceutical Compliance Software Market Size by Type (2022-2027)
6.2.3 North America Pharmaceutical Compliance Software Market Size by Type (2017-2027)
6.3 North America Pharmaceutical Compliance Software Market Size by Application
6.3.1 North America Pharmaceutical Compliance Software Market Size by Application (2017-2022)
6.3.2 North America Pharmaceutical Compliance Software Market Size by Application (2022-2027)
6.3.3 North America Pharmaceutical Compliance Software Market Size by Application (2017-2027)
6.4 North America Pharmaceutical Compliance Software Market Size by Country
6.4.1 North America Pharmaceutical Compliance Software Market Size by Country (2017-2022)
6.4.2 North America Pharmaceutical Compliance Software Market Size by Country (2022-2027)
6.4.3 United States
6.4.4 Canada
7 Europe
7.1 Europe Pharmaceutical Compliance Software Market Size (2017-2027)
7.2 Europe Pharmaceutical Compliance Software Market Size by Type
7.2.1 Europe Pharmaceutical Compliance Software Market Size by Type (2017-2022)
7.2.2 Europe Pharmaceutical Compliance Software Market Size by Type (2022-2027)
7.2.3 Europe Pharmaceutical Compliance Software Market Size by Type (2017-2027)
7.3 Europe Pharmaceutical Compliance Software Market Size by Application
7.3.1 Europe Pharmaceutical Compliance Software Market Size by Application (2017-2022)
7.3.2 Europe Pharmaceutical Compliance Software Market Size by Application (2022-2027)
7.3.3 Europe Pharmaceutical Compliance Software Market Size by Application (2017-2027)
7.4 Europe Pharmaceutical Compliance Software Market Size by Country
7.4.1 Europe Pharmaceutical Compliance Software Market Size by Country (2017-2022)
7.4.2 Europe Pharmaceutical Compliance Software Market Size by Country (2022-2027)
7.4.3 Germany
7.4.4 France
7.4.5 U.K.
7.4.6 Italy
7.4.7 Russia
7.4.8 Nordic
8 Asia-Pacific
8.1 Asia-Pacific Pharmaceutical Compliance Software Market Size (2017-2027)
8.2 Asia-Pacific Pharmaceutical Compliance Software Market Size by Type
8.2.1 Asia-Pacific Pharmaceutical Compliance Software Market Size by Type (2017-2022)
8.2.2 Asia-Pacific Pharmaceutical Compliance Software Market Size by Type (2022-2027)
8.2.3 Asia-Pacific Pharmaceutical Compliance Software Market Size by Type (2017-2027)
8.3 Asia-Pacific Pharmaceutical Compliance Software Market Size by Application
8.3.1 Asia-Pacific Pharmaceutical Compliance Software Market Size by Application (2017-2022)
8.3.2 Asia-Pacific Pharmaceutical Compliance Software Market Size by Application (2022-2027)
8.3.3 Asia-Pacific Pharmaceutical Compliance Software Market Size by Application (2017-2027)
8.4 Asia-Pacific Pharmaceutical Compliance Software Market Size by Country
8.4.1 Asia-Pacific Pharmaceutical Compliance Software Market Size by Country (2017-2022)
8.4.2 Asia-Pacific Pharmaceutical Compliance Software Market Size by Country (2022-2027)
8.4.3 China
8.4.4 Japan
8.4.5 South Korea
8.4.6 Southeast Asia
8.4.7 India
8.4.8 Australia
9 Latin America
9.1 Latin America Pharmaceutical Compliance Software Market Size (2017-2027)
9.2 Latin America Pharmaceutical Compliance Software Market Size by Type
9.2.1 Latin America Pharmaceutical Compliance Software Market Size by Type (2017-2022)
9.2.2 Latin America Pharmaceutical Compliance Software Market Size by Type (2022-2027)
9.2.3 Latin America Pharmaceutical Compliance Software Market Size by Type (2017-2027)
9.3 Latin America Pharmaceutical Compliance Software Market Size by Application
9.3.1 Latin America Pharmaceutical Compliance Software Market Size by Application (2017-2022)
9.3.2 Latin America Pharmaceutical Compliance Software Market Size by Application (2022-2027)
9.3.3 Latin America Pharmaceutical Compliance Software Market Size by Application (2017-2027)
9.4 Latin America Pharmaceutical Compliance Software Market Size by Country
9.4.1 Latin America Pharmaceutical Compliance Software Market Size by Country (2017-2022)
9.4.2 Latin America Pharmaceutical Compliance Software Market Size by Country (2022-2027)
9.4.3 Mexico
9.4.4 Brazil
10 Middle East & Africa
10.1 Middle East & Africa Pharmaceutical Compliance Software Market Size (2017-2027)
10.2 Middle East & Africa Pharmaceutical Compliance Software Market Size by Type
10.2.1 Middle East & Africa Pharmaceutical Compliance Software Market Size by Type (2017-2022)
10.2.2 Middle East & Africa Pharmaceutical Compliance Software Market Size by Type (2022-2027)
10.2.3 Middle East & Africa Pharmaceutical Compliance Software Market Size by Type (2017-2027)
10.3 Middle East & Africa Pharmaceutical Compliance Software Market Size by Application
10.3.1 Middle East & Africa Pharmaceutical Compliance Software Market Size by Application (2017-2022)
10.3.2 Middle East & Africa Pharmaceutical Compliance Software Market Size by Application (2022-2027)
10.3.3 Middle East & Africa Pharmaceutical Compliance Software Market Size by Application (2017-2027)
10.4 Middle East & Africa Pharmaceutical Compliance Software Market Size by Country
10.4.1 Middle East & Africa Pharmaceutical Compliance Software Market Size by Country (2017-2022)
10.4.2 Middle East & Africa Pharmaceutical Compliance Software Market Size by Country (2022-2027)
10.4.3 Turkey
10.4.4 Saudi Arabia
10.4.5 UAE
11 Key Players Profiles
11.1 Wolters Kluwer
11.1.1 Wolters Kluwer Company Details
11.1.2 Business Overview and Recent Development
11.1.3 Pharmaceutical Compliance Software Introduction
11.1.4 Wolters Kluwer Value in Pharmaceutical Compliance Software Business (2017-2022)
11.2 ACUTA
11.2.1 ACUTA Company Details
11.2.2 Business Overview and Recent Development
11.2.3 Pharmaceutical Compliance Software Introduction
11.2.4 ACUTA Value in Pharmaceutical Compliance Software Business (2017-2022)
11.3 Bwise
11.3.1 Bwise Company Details
11.3.2 Business Overview and Recent Development
11.3.3 Pharmaceutical Compliance Software Introduction
11.3.4 Bwise Value in Pharmaceutical Compliance Software Business (2017-2022)
11.4 Qordata
11.4.1 Qordata Company Details
11.4.2 Business Overview and Recent Development
11.4.3 Pharmaceutical Compliance Software Introduction
11.4.4 Qordata Value in Pharmaceutical Compliance Software Business (2017-2022)
11.5 Lachman Consultant Services
11.5.1 Lachman Consultant Services Company Details
11.5.2 Business Overview and Recent Development
11.5.3 Pharmaceutical Compliance Software Introduction
11.5.4 Lachman Consultant Services Value in Pharmaceutical Compliance Software Business (2017-2022)
11.6 Ideagen
11.6.1 Ideagen Company Details
11.6.2 Business Overview and Recent Development
11.6.3 Pharmaceutical Compliance Software Introduction
11.6.4 Ideagen Value in Pharmaceutical Compliance Software Business (2017-2022)
11.7 MasterControl
11.7.1 MasterControl Company Details
11.7.2 Business Overview and Recent Development
11.7.3 Pharmaceutical Compliance Software Introduction
11.7.4 MasterControl Value in Pharmaceutical Compliance Software Business (2017-2022)
11.8 Sparta Systems
11.8.1 Sparta Systems Company Details
11.8.2 Business Overview and Recent Development
11.8.3 Pharmaceutical Compliance Software Introduction
11.8.4 Sparta Systems Value in Pharmaceutical Compliance Software Business (2017-2022)
11.9 Intagras
11.9.1 Intagras Company Details
11.9.2 Business Overview and Recent Development
11.9.3 Pharmaceutical Compliance Software Introduction
11.9.4 Intagras Value in Pharmaceutical Compliance Software Business (2017-2022)
11.10 LogicGate
11.10.1 LogicGate Company Details
11.10.2 Business Overview and Recent Development
11.10.3 Pharmaceutical Compliance Software Introduction
11.10.4 LogicGate Value in Pharmaceutical Compliance Software Business (2017-2022)
12 Analyst’s Viewpoints/Conclusions
13 Appendix
13.1 Methodology
13.2 Research Data Source
13.2.1 Secondary Data
13.2.2 Primary Data
13.2.3 Market Size Estimation
13.2.4 Legal Disclaimer
